Background: Diamond Blackfan anemia (DBA) is a rare inherited bone marrow failure syndrome characterized by anemia, congenital anomalies and a predisposition to cancer. Patients usually present during infancy or early childhood, but can also be diagnosed as adults. In the vast majority of cases DBA is due to a mutation in a gene encoding a small or large subunit-associated ribosomal protein (RP) leading to RP haploinsufficiency. In a study of 702 patients enrolled in the DBA Registry (DBAR), the observed to expected ratio for acute myeloid leukemia (AML) was 28.8 and for myelodysplastic syndrome (MDS), 352.1 (Vlachos et al, Blood, 2018). The average age of onset for MDS in the DBA cohort was 26 years, compared to 60-70 years in the general population. Evolving clonal hematopoiesis (CH) with age has been observed as a precursor to MDS, with CH rarely observed in individuals younger than 40 years of age. Thus we hypothesized that the young age at the development of MDS in DBA would be presaged by evolving CH.
Objective: The primary objective was to perform whole exome sequencing (WES) specifically screening for previously reported somatic mutations in 56 genes associated with CH (Jaiswal et al, NEJM, 2014).
Design/Method: A total of 69 samples were analyzed from 65 patients, mostly targeting patients older than 18 years (median age 30 years). Multiple samples were run on patients who had available samples in the DBAR Biorepository to determine rate of acquisition of mutations. 468 age- and sex-matched healthy controls were made available from GeneDx who performed the WES for the study. We used a threshold for variant calling of minimum 5% with a minimum of 2 variant reads.
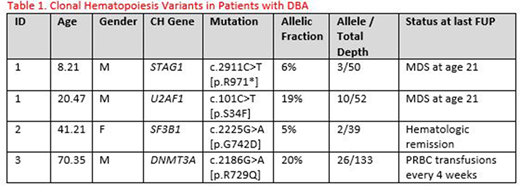
Results: Three of the 65 DBA patients (5%) were found to have somatic mutations in STAG1, U2AF1, SF3B1, and DNMT3A at 8, 20, 41, and 70 years, respectively (Table 1). The patient who was 20 years of age had a sample in the DBAR biorepository from when he was age 8 years which was found to have a different somatic mutation (STAG1) than was found at present (U2AF1). This patient did go on to develop MDS at the age of 21 years. In comparison, of the 468 controls, 4 (0.8 %) had a somatic mutation in SF3B1, LUC7L2, DNMT3A, and LUC7L2 at ages 12, 31, 33 and 40 years, respectively.
Conclusion: Patients with DBA show more somatic mutations as compared to controls (p<0.05). This early acquisition of mutations may be the driving force for their developing MDS at an earlier age than that of the general population. Further studies with more sensitive methods are warranted to accurately determine the prevalence of somatic CH mutations and their potential association with the development of myelodysplastic syndrome in these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal